Natural Coulomb Electrostatics (NCE) Analysis
-
Reference
F. Weinhold and C. R. Landis, Discovering Chemistry with Natural Bond Orbitals (Wiley, 2012), Sec. 6.2.
-
Notes
A superficial picture of long-range electronic interactions envisions effective "atomic point charges" QA at each nuclear center interacting according to the classical potential energy of Coulomb electrostatics (CE):
ECE = ΣA,B QA QB/RAB
Although totally inadequate in the domain of exchange interactions, this formula may provide useful estimates of "electrostatic effects" at longer range (RAB > sum of atomic van der Waals radii). When evaluated with natural atomic charges, the formula defines what may be called the "Natural Coulomb Electrostatics" (ENCE) potential energy for the species and geometry in question. Variations of ENCE with respect to geometry changes provide simple estimates of electrostatic contributions to intra- or intermolecular interaction energy, supplementing independent estimates of steric and donor-acceptor contributions for comparison with more detailed "energy component" analyses (e.g., NEDA). From the known NBO charge distributions in the idealized NLS, one can also evaluate separate Lewis (L) and non-Lewis (NL) contributions to NPA charge, together with corresponding L/NL contributions to ENCE that allow estimates of non-classical NL-induced (resonance type) charge shifts on overall dielectric properties. These NL-induced charge shifts are often found to be leading contributors to chemically important "electrostatic effects", providing an important quantal correction to quasi-classical interpretation of total ENCE. For open shells, an additional spin-charge NCE table shows the distinct α-NCE and β-NCE contributions of each spin set, again emphasizing the limitations of naive classical interpretations.NCE keyword results are illustrated for a model water dimer at RHF/6-311++G** level , showing intra- and intermolecular NCE values between the two monomer units. (see NBO 6.0 Manual, pp. B?-? for additional discussion).
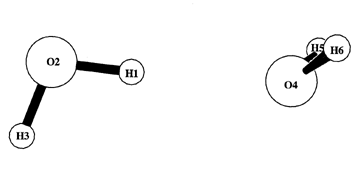
-
Sample Gaussian Input
%chk=h2o_h2o
#N rhf/6-311++G** POP=NBORead
H2O...HOH
0 1
H 0.036726 0.556520 0.000000
O -0.006183 1.525447 0.000000
H 0.909465 1.817564 0.000000
O -0.006183 -1.376675 0.000000
H -0.423629 -1.782131 0.767110
H -0.423629 -1.782131 -0.767110
$NBO nce $END